
Wuhan Dgensee
武汉臻熙
Who we are
P.S. Dgensee is a homonym for Cherish in ChineseWuhan Dgensee was founded in 2018 and is located on the fifth floor of Building B21, Wuhan Optics Valley Medical Device Park, East Lake New Technology Development Zone, Wuhan, China. It is a high-tech start-up company focusing on the application of gene technology to the detection of pathogens in clinic. Relying on gene technology and with targeted sequencing technology as a means, the company is committed to in-depth research and development as well as interdisciplinary innovation. It has continuously developed and established scientific and standard detection methods and technical platforms in sample extraction, amplification, database establishment, and sequencing; promoted intelligence in data analysis; and finally submitted the standardised, easily operable, and widely applied pathogenetic diagnostic kits for approval. The perfect combination of the advantages of rapid and accurate gene technology will promote the iterative upgrading of the clinical diagnostic industry, overturn the entire microbial testing industry, and provide more effective clinical options for clinicians and patients.
The Fourth Generation of Gene Sequencing Technology Leader
Since 2012, the core members of the Wuhan Dgensee team have been committed to the application development and product transformation of third-generation sequencing technology, original focus on PacBio sequencing, and then making Wuhan Dgensee the first company in China to explore and transform the clinical application of ONT products in 2018. Based on the ONT sequencing platform, we have carried out the testing and validation of whole genomics, metagenomics, capture sequencing genomics, targeted amplification genomics, and other sequencing methodologies and applications. Currently, in the field of ONT sequencing of pathogenic infections, the company has compared and analysed a variety of methodologies for clinical diagnosis of infections caused by potential pathogens in parallel. Combining this with the existing technology and data characteristics of nanopore sequencing technology as well as the expected future technological innovation, it has conducted an in-depth evaluation from the perspectives of accuracy, sensitivity, and clinical applicability and has finally launched a suitable clinical pathogen diagnostic protocol with nanopore sequencing, which named as nanopore targeted sequencing (NTS). The advantages of the protocol are provided below.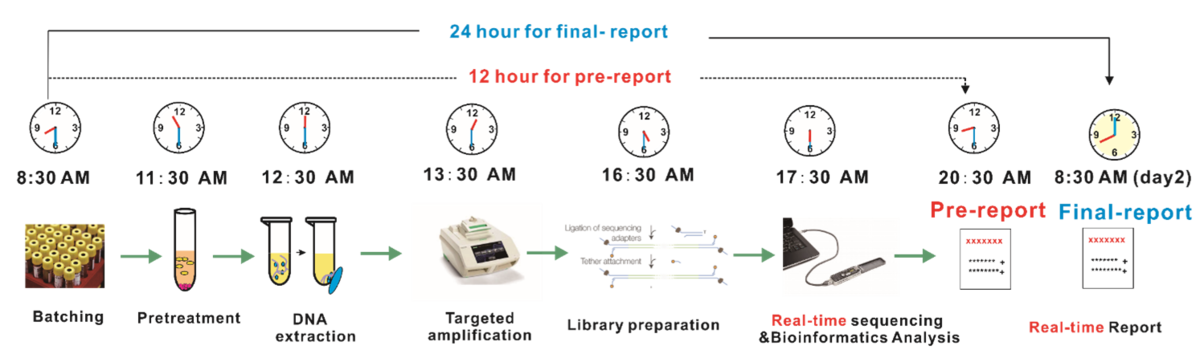 Figure 1. Turnaround time for the NTS protocolSpeed is one of the most critical factors in the diagnosis of infectious diseases. Currently, NTS is already able to achieve 12–24 h turnaround time from receiving samples to issuing reports in clinical practice (Figure 1), which is expected to be reduced to 12–16 h, achieving same-day issuance of test reports.
Figure 1. Turnaround time for the NTS protocolSpeed is one of the most critical factors in the diagnosis of infectious diseases. Currently, NTS is already able to achieve 12–24 h turnaround time from receiving samples to issuing reports in clinical practice (Figure 1), which is expected to be reduced to 12–16 h, achieving same-day issuance of test reports.We developed a protocol for the detection of novel coronavirus using NTS technology, which was published as a cover article in the high-impact academic journal Small (Figure 2), and our first version of NTS protocol for pathogen detection has published in MedRxiv.
Media Articles
Contact Us

No.2, 5th floor, building B21, 818 Gaoxin Avenue, Donghu New Technology Development Zone, Wuhan
027-63499663
+86 13797009319
hr@dgensee.com
© 2021




 鄂公网安备 42018502005148号
鄂公网安备 42018502005148号